Definitions

There are several measures of aerosol concentration. The most important in the area of environmental science and health is the mass concentration (M), defined as the mass of particulate matter per unit volume with units such as μg/m3. Also commonly used is the number concentration (N), the number of particles per unit volume with units such as number/m3 or number/cm3.[5]
The size of particles has a major influence on their properties and the aerosol particle radius or diameter (dp) is a key property used to characterise aerosols. If all the particles in an aerosol are the same size it is known as monodisperse and this type of aerosol can be produced in the laboratory. Most aerosols however are polydisperse, i.e. they have a range of particle sizes.[6] While liquid droplets are nearly always spherical, solid particles have a variety of shapes and to understand their properties, a equivalent diameter is used. The equivalent diameter is the diameter of a regular particle which has the same value of some physical property as the irregular particle.[7] The equivalent volume diameter (de) is defined as the diameter of a sphere having the same volume as that of the irregular particle.[8] Also commonly used is the aerodynamic diameter.
[edit] Size distribution

 is the diameter of the particles
is the diameter of the particles is the fraction of particles having diameters between
is the fraction of particles having diameters between  and
and  +
+ 
 is the frequency function
is the frequency function
A more widely chosen distribution is the log-normal distribution where the number frequency is given as:
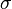 is the standard deviation of the size distribution and
is the standard deviation of the size distribution and is the arithmetic mean diameter.
is the arithmetic mean diameter.
Other distributions which can be used to characterise particle size are: the Rosin-Rammler distribution, applied to coarsely dispersed dusts and sprays; the Nukiyama-Tanasawa distribution, for sprays having extremely broad size ranges; the power function distribution, which has been applied to atmospheric aerosols; the exponential distribution, applied to powdered materials and for cloud droplets the Khrgian-Mazin distribution.
[edit] Physics
[edit] Terminal velocity of a particle in a fluid
For low values of the Reynolds number (<1), which is true of most aerosol motion, Stokes' law describes the force of resistance on a solid spherical particle in a fluid. However, Stoke's law is only valid when the velocity of the gas at the particle's surface is zero. For small particles (< 1 μm) this assumption breaks down. This can be accounted for by introducing the Cunningham correction factor which is always greater than 1. Including this factor we find that the relation between the resisting force on a particle and its velocity is: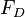 is the resisting force on a spherical particle
is the resisting force on a spherical particle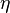 is the viscosity of the gas
is the viscosity of the gas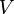 is the particle velocity
is the particle velocity is the Cunningham correction factor.
is the Cunningham correction factor.
 is the terminal settling velocity of the particle.
is the terminal settling velocity of the particle.

If a particle is travelling a velocity other than its terminal velocity it approaches the terminal velocity exponentially with an e-folding time equal to the relaxation time:
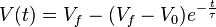
where:
 is the particle speed at time t
is the particle speed at time t is the final particle speed
is the final particle speed is the initial particle speed
is the initial particle speed

where:
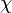 is the dynamic shape factor
is the dynamic shape factor
[edit] Aerodynamic diameter
The aerodynamic diameter of an irregularly shaped particle is defined as the diameter of the spherical particle with a density of 1000 kg/m3 that has the same settling velocity as the irregular particle.[22]Neglecting the slip correction, the terminal velocity at which the particle settles is proportional to the square of the aerodynamic diameter, da:[22]
 = standard particle density (1000 kg/m3).
= standard particle density (1000 kg/m3).
[edit] Dynamics
The previous discussion focussed on single aerosol particles. In contrast, aerosol dynamics explains the evolution of complete aerosol populations. The concentrations of particles will change over time as a result of many processes. External processes which move particles across the wall of a volume of gas include diffusion, gravitational settling and migration caused by external forces such as electric charges. A second set of processes are internal to a given volume of gas are particle formation (nucleation), evaporation or chemical reaction and coagulation.[24]The evolution of the aerosol due to these process can be characterized by a differential equation called the Aerosol General Dynamic Equation (GDE).[24]
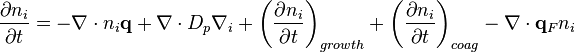
Change in time = Convective transport + brownian diffusion + gas-particle interactions + coagulation + migration by external forces
Where:
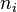 is number density of particles of size category
is number density of particles of size category 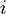
 is the particle velocity
is the particle velocity is the particle Stokes-Einstein diffusivity
is the particle Stokes-Einstein diffusivity is the particle velocity associated with an external force
is the particle velocity associated with an external force
[edit] Coagulation
When particles are present in an aerosol they collide with each other. During that they may undergo coalescence or aggregation. This process leads to a change in the aerosol number/size distribution function, with the mode growing in diameter and decreasing in number.[edit] Dynamics regimes
There are three different dynamical regimes which govern the behaviour of an aerosol, which can be defined by the Knudsen number of the particle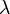 is the mean free path of the suspending gas and
is the mean free path of the suspending gas and  is the diameter of the particle. Particles are in the free molecular regime when Kn >> 1, that is particles are small compared to the mean free path of the suspending gas.
In this regime, particles interact with the suspending gas through a
series of 'ballistic' collisions with gas molecules. As such, they
behave similarly to gas molecules, tending to follow streamlines and
diffusing rapidly through Brownian motion. The mass flux equation in
the free molecular regime is:
is the diameter of the particle. Particles are in the free molecular regime when Kn >> 1, that is particles are small compared to the mean free path of the suspending gas.
In this regime, particles interact with the suspending gas through a
series of 'ballistic' collisions with gas molecules. As such, they
behave similarly to gas molecules, tending to follow streamlines and
diffusing rapidly through Brownian motion. The mass flux equation in
the free molecular regime is:Particles are in the continuum regime when Kn << 1. In this regime, the particles are big compared to the mean free path of the suspending gas, meaning that the suspending gas can be though of as a continuous fluid flowing round the particle. The molecular flux in this regime is:
The transition regime contains all the particles in between the free molecular and continuum regimes or Kn ≈ 1. The forces experienced by a particle are a complex combination of interactions with individual gas molecules, and macroscopic interactions. The semi-empirical equation describing mass flux is:
[edit] Partitioning

- If the vapour pressure is lowered by lowering the temperature of the vapour
- If chemical reactions increase the partial pressure of a gas, or lower its vapour pressure
- If the addition of another vapour lowers the equilibrium vapour pressure due to the Raoult Effect
[edit] Activation
Aerosols are said to be activated when they become coated by water, usually in the context of forming a cloud droplet[citation needed]. Following the Kelvin effect (based on the curvature of liquid droplets) smaller particles need a higher ambient relative humidity to maintain equilibrium than bigger ones would. Relative humidity (%) for equilibrium can be determined from the following formula: is the saturation vapor pressure above a particle at equilibrium (around a curved liquid droplet), p0 is the saturation vapor pressure (flat surface of the same liquid) and S is the saturation ratio.
is the saturation vapor pressure above a particle at equilibrium (around a curved liquid droplet), p0 is the saturation vapor pressure (flat surface of the same liquid) and S is the saturation ratio.Kelvin equation for saturation vapor pressure above a curved surface is:
[edit] Solution to the General Dynamic Equation
There are no general solutions to the General Dynamic Equation (GDE) - common methods used to solve the GDE are:[citation needed]- Moment method
- Modal/Sectional Method
- Quadrature Method of Moments.















Tidak ada komentar:
Posting Komentar